Frequently Asked Questions on Permeation
- wiphaweetnvbow
- May 30, 2025
- 2 min read
Updated: Jun 2, 2025
Source: AMETEK MOCON Website - Knowledge
1. What Is Permeation?
Permeation is the movement of gases or vapors through a barrier material, such as a bottle wall or film layer. It occurs naturally, moving from areas of higher concentration to areas of lower concentration.

Example: In a freshly filled bottle of carbonated soft drink, the CO₂ concentration inside (~4 atm) is much higher than the CO₂ in the surrounding air (<0.5%). Over time, CO₂ permeates out, while oxygen from the air moves into the bottle. This change leads to product degradation, such as a “flat” taste.

Permeation Occurs in 3 Steps (Solution-Diffusion Mechanism):
Molecules are absorbed into the high-concentration surface
Molecules diffuse through the material
Molecules desorb on the low-concentration side
Permeation is influenced by:
Solubility (S): How well the gas dissolves in the material
Diffusivity (D): How easily the gas moves through the material
Key Equation:
Permeability (P) = Diffusion Coefficient (D) × Solubility Coefficient (S)
In practice, Transmission Rate (TR) is used to report the amount of gas that passes through a material:
OTR (Oxygen): cc/m².day
WVTR (Water Vapor): g/m².day
CO₂TR (Carbon Dioxide): cc/m².day
2. How Is Permeation Measured?
Permeation is measured using transmission rate tests under controlled conditions. A common setup (Iso-static method) places test gas on one side of a film and a carrier gas (usually nitrogen) on the other. The permeated gas is carried to a sensor for analysis.
Common Instruments:
OX-TRAN® 2/22 – Measures OTR using Coulometric sensors
AQUATRAN® 3/34 – Measures WVTR
PERMATRAN-C® – Measures CO₂TR
3. What Factors Influence Permeation?
Environmental factors:
Temperature: Every 10°C increase doubles transmission rate
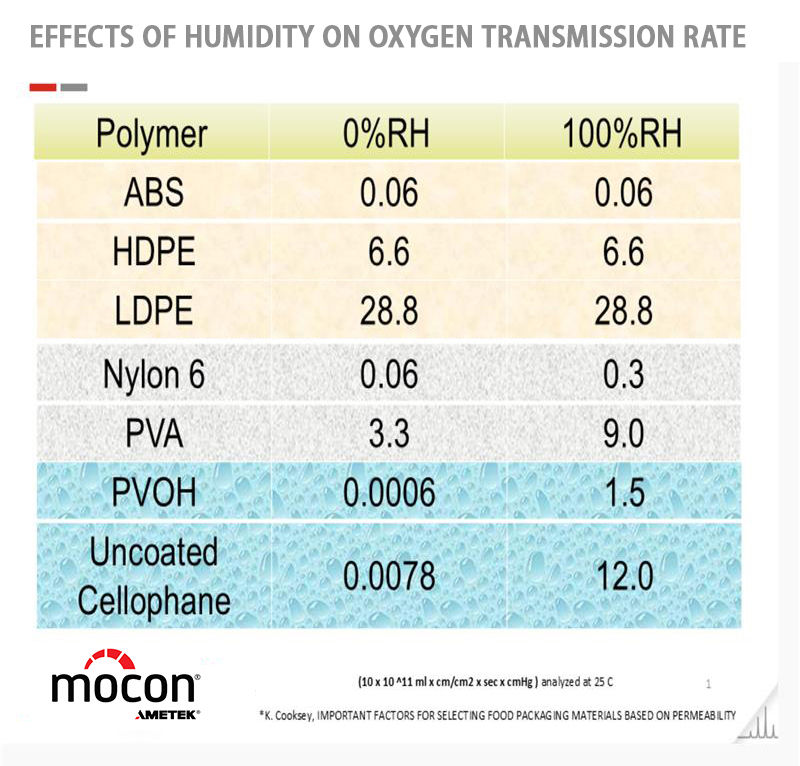
Relative Humidity (RH): Affects hydrophilic materials (e.g., EVOH)
Driving Force: Higher pressure difference increases permeation
Material Thickness: Thinner materials = faster permeation
Material properties:
Polymer chain structure and crystallinity
Moisture interaction and polarity
Surface characteristics and additives
4. ASTM D3985 vs F2622: Which OTR Method Should You Use?
Oxygen barrier packaging is essential for oxygen-sensitive products. Accurate OTR testing ensures proper material selection and quality assurance.
Two ASTM Methods:
D3985: Uses Coulometric sensor (no calibration needed)
F2622: Uses non-Coulometric sensors (requires calibration)
Comparison Table:
Feature | Coulometric (D3985) | Non-Coulometric (F2622) |
Sensor calibration needed | No | Yes |
Carrier gas dependency | No | Yes |
Sensor response linearity | Linear | Not linear |
Best for | High-barrier films | Medium/low barrier films |
Sensor cost | Higher | Lower |
NIST traceable | Yes | No |
Recommendation:
High-barrier materials (e.g., for sensitive foods): Use Coulometric method for higher accuracy and reliability
Low-barrier materials (e.g., fruit packaging): Non-Coulometric method is suitable
5. Should I Test OTR with Specific Relative Humidity (RH)?
Yes, depending on the type of polymer.
Fickian Materials (e.g., polyolefins): Hydrophobic, OTR results are consistent regardless of RH
Non-Fickian Materials (e.g., EVOH): Hydrophilic, RH affects polymer structure and permeability